Crystal structure, EMPA and FTIR spectroscopy of green yoderite: (Mg2.02Al5.68Fe3+0.35)Σ8.05 (Si3.93 P5+0.03)Σ3.96O18(OH)2; from Mautia Hill, Tanzania
DOI:
https://doi.org/10.13133/2239-1002/17556Abstract
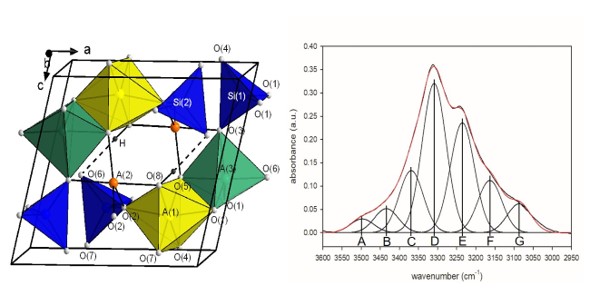
Green yoderite, with the ideal crystal-chemical formula [6](MgAl3)[5](MgAl) [5](Al2)O2(SiO4)4(OH)2, from the type locality, Mautia Hill, Tanzania, has been characterized by accurate X-ray diffraction data and crystal-structure refinement at ambient conditions, electron microprobe analysis and FTIR spectroscopy. Yoderite is monoclinic with a = 7.9979 (3), b = 5.8003 (2), c = 7.2299 (2), ß= 104.7398 (9) Å, V = 324.36 (2) ų, space group P21/n (no. 14), Z = 1. Crystal-structure refinement with all non-H atoms refined anisotropically converged to an R1 value of 2.50 % based on all 2156 unique reflections and 94 refined parameters. Chains of edge-sharing A(1)O5(OH) octahedra extend in the b direction and the A(1) site is occupied by 0.75 Al + 0.25 Mg. These chains are linked by isolated SiO4 tetrahedra and by two independent trigonal bipyramids A(2)O4(OH) and A(3)O5, where the A(2) site is occupied by 0.50 Al + 0.50 Mg and the A(3) site by 0.94 Al + 0.06 Fe3+. The hydrogen-bond scheme is established on the basis of the H-atom position observed in the final ΔF map and refined without restraint. Fitting of the FTIR spectrum in the principal (OH)-stretching region shows that A(2)Al and A(2)Mg are preferentially associated with certain A(1)(AlAl), A(1)(AlMg) and A(1)(MgMg) dimers. Comparison of the band positions, observed intensities and associated local short-range arrangements suggests that the bond valence requirements of such arrangements control the chemical composition of the crystal.
Downloads
Published
Issue
Section
License
Copyright (c) 2021 Periodico di Mineralogia

This work is licensed under a Creative Commons Attribution 4.0 International License.

